Bacteriocins
Bacteriocins are narrow-spectrum, protein antibiotics produced by bacteria which kill closely related species; for example colicins are bacteriocins produced by E.coli which kill E.coli strains and Salmonella. Bacteriocins are produced by all the major lineages of bacteria and have evolved over 3 million years. In comparison mankind has been discovering and developing antibiotics for only 80 years. The activity of bacteriocins against Gram-negative bacterial species is potentially of great value given the current paucity of therapeutic option for treating such infections for treating such infections.
Bacteriocins are multidomain proteins consisting of;
- a killing domain which is responsible for killing the sensitive bacterial cell; examples include DNAses, RNAses and pore-forming killing domains
- a receptor-binding domain that allows binding to a specific receptor on bacterial cells
- a translocation domain that after cell-binding facilitates delivery of the killing domain to its cellular target
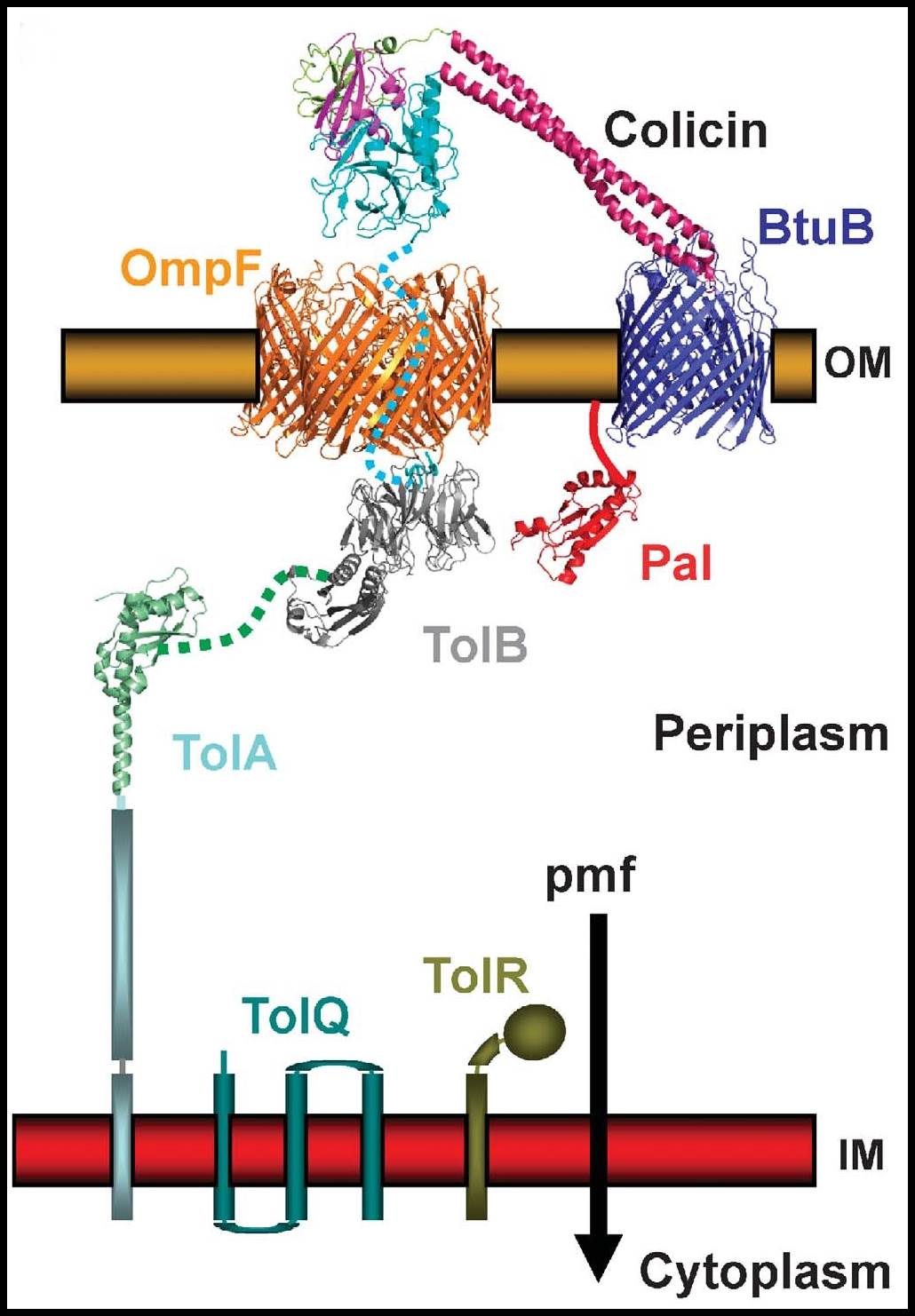
Figure; Model of the delivery of colicin E3 into an E.coli cell. Colicin E3 has an RNase killing domain that must be delivered into the cytoplasm of an E.coli cell in order to kill. The helical hairpin receptor-binding domain of colicin E3 binds to the BtuB receptor in the outer membrane of the E.coli cell. The RNase domain then crosses the outer membrane via the OmpF porin, in a process that requires interactions of the translocation domain with the TolB and TolA proteins in the periplasm. Finally entry of the RNase domain into the cytoplasm requires energy provided by the pmf.
Identifying bacteriocin markets
A number of markets are envisaged for bacteriocin antibiotics;
- Treatment of vegetables and fresh meat such as beef, pork, chicken and turkey
- Veterinary use to prevent infections such as mastitis in cows and diarrhoea in pigs
- Therapeutic use in patients to treat serious bacterial infections
- A platform technology to develop a range of novel therapeutics such as antibacterial, antifungal, antiviral, and anti-cancer drugs
The regulatory requirements will be more complex for veterinary or therapeutic use but it is likely that bacteriocin treatment of vegetables and meat will begin soon using native bacteriocin proteins that are identical to those produced by bacteria. Three clinical trials are planned in the next few years to test the efficacy of native bacteriocins to treat important infections.
Bacteriocin-derived antibiotics
It is possible to engineer novel bacteriocins with a different spectrum of killing by swapping the domains of native bacteriocins. These bacteriocin-derived antibiotics (BDAs) have considerable potential as narrow-spectrum antibiotics for veterinary and therapeutic use. One class of BDAs are the Pheromonicins being developed in China by Dr X-Q Qiu. Pheromonicins are based upon the killing activity of colicin Ia, a bacteriocin which forms pores in the cytoplasmic membrane of E.coli leading to cell death. Preliminary data indicates that fusion of colicin Ia to a range of different targeting domains can result in a range of antibacterial, antifungal, antiviral, and even anti-cancer drugs (link). If the potential of BDAs is confirmed in different laboratories then the landscape for antibiotic discovery could be dramatically changed