Bacteriophage therapy
Bacteriophage (phage) are bacterial viruses that kill bacteria by binding to a surface receptor of a bacterial cell, inject DNA or RNA into the cell, which is replicated and produces viral proteins. Phage nucleic acid is then packaged into assembled viral coat proteins to make 10-200 active phage which are released from the bacterial cell, killing it in the process. The released phage can then bind to nearby bacterial cells and repeat the process, ensuring an amplification in phage numbers whilst bacterial killing takes place. This is the principle of bacteriophage therapy (phage therapy) of infections.
Phage can be classified based on their;
- Morphology - the majority of phage are tailed (as in the figure below) but some are polyhedral, filamentous or pleomorphic
- Nucleic acid content - DNA or RNA
- Specific host - staphylococci, Escherichia coli etc.
- Life cycle - the lytic cycle where enzymes encode by the phage nucleic acid promote lysis of the host bacterium and release of phage particles, and the lysogenic cycle where the phage DNA is inserted into the host bacterial chromosome and replicates along with it until the lytic cycle is induced
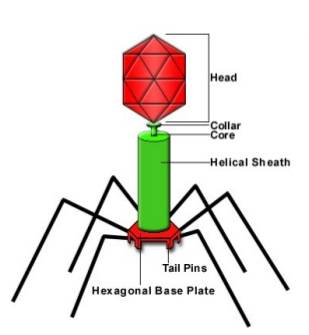
Figure. Structure of a tailed bacteriophage
Early History of Phage Therapy
The first paper describing the clinical use of phage therapy was published in 1921 by Bruynoghe & Maisin who used a staphylococcal-specific phage to treat cutaneous furuncles and carbuncles. The development of electron microscopes resulted in the first description of phage as sperm-shaped particles by Ruska in Germany in 1940. Questions over the scientific rigour of the published phage therapy reports, together with the beginning of the golden age of antibiotic discovery, reduced interest in bacteriophage therapy except in the Soviet Union and in Poland.
Smith & Huggins in England carried out important experiments using phage therapy to treat E.coli infections in mice, calves, piglets and lambs in 1982 and 1983. The first phase 1 randomized controlled trial conducted in the USA by Rhoads et al. was published in 2009 and evaluated the safety of a cocktail of phages in 42 patients with chronic venous leg ulcers. Another randomized controlled clinical trial that studied the efficacy of a cocktail of phages in treating chronic Pseudomonas aeruginosa otitis was also published in England in 2009.
The U.S. National Institutes of Health in June 2016 lists four Clinical Trials involving phage therapy, two of which have been completed (to treat cystic fibrosis or venous leg ulcers), one is recruiting (the Phagoburn project to treat E.coli and P.aeruginosa in burned patients) and the other (to treat diabetic foot ulcers) is not yet recruiting.
In principle phage therapy has significant promise in being able to treat MDR infections and help alleviate the crisis caused by antimicrobial resistance in pathogens, however the potential advantages and disadvantages of phage therapy have to be considered in detail.
Advantages of Phage Therapy
- Phage are natural antimicrobials that are active against Gram-positive or Gram-negative infections, including MDR pathogens
- Phage are narrow-spectrum with activity limited to a single species, or in some case a single strain of one species. this reduces the collateral damage to other bacteria in the microbiome
- Phage numbers are amplified during bacterial killing
- Excellent tolerability has been reported in animal models and in healthy human volunteers
- Wide distribution of phages including crossing the blood-brain barrier after systemic administration
- Some phage can attack bacterial biofilms
- Phage therapy may also have a beneficial impact on the inflammatory response to infection
- The costs of bacteriophage therapy may be lower than antibiotic treatment
Limitations of Phage Therapy
- The extreme narrow-spectrum of phage means that the infectious organism needs to be identified to allow selection of the appropriate phages to use to treat the infection
- Phage resistance is common in the target bacteria due to for example mutation or loss of the bacteriophage receptor on the cell surface. This problem is usually overcome by using a cocktail of different phages that bind to differect receptors in the same bacterial species, however a recent success with phage monotherapy against Pseudomonas aeruginosa is eencouraging (link)
- The optimal dose, frequency and duration of treatment needs to be determined
- Phages can be recognized by the immune system and may be rapidly eliminated from the systemic circulation
- Potential egulatory problems with phage product quality and safety requirements, especially those related to producing a consistent cocktail of phages
- IP protection may be difficult to obtain for phage therapy
- The clinical acceptability of phage technology
A Cautionary Experience
It is hoped that evidence supporting the clinical value of phage therapy will result from some of the clinical trials that have been completed or are in progress, however the following experience provides a warning of some of the pitfalls that can arise.
Diarrhoea-associated E.coli was chosen for a proof-of-concept clinical trial of phage therapy led by Sarker and Brussow that took over 20 years. Many T4-like phages were isolated from patients with diarrhoea based on their ability to kill a specific E.coli K12 strain. Cocktails of at least 10 different T4-like phage isolates were needed to be able to kill 50% of a large collection of E.coli pathogenic strains from Mexico and Bangladesh.
Production, stability and safety tests were then carried out with the cocktail of phages, followed by Phase I animal and then human safety tests. Finally the T4 phage cocktail was tested in a Phase II randomized, placebo-controlled single centre trial in Bangladeshi children hospitalized with acute E.coli diarrhoea. Unfortunately the results of the trial showed that the oral phage cocktail did not perform better than oral rehydration/zinc treatment. Analysis of the stools of the children revealed the presence of mixed infections, often of five different pathogens. Analysis of this data suggested that faecal streptococci could be causing the diarrhoea rather than E.coli, thus providing an explanation for the apparent failure of bacteriophage therapy.
A Hopeful Experience
The story of the use of phage therapy to save the life of a Professor of Psychiatry at the University of California, San Diego is heart warming and hopefully a sign of the potential of phage therapy in treating life-threatening infections (link).
